StreamLiner Use Case
Endovascular Interventions Below-the-Knee
The mainstay of endovascular treatment below-the-knee is typically balloon angioplasty, atherectomy, and thrombectomy, followed by stenting in…
The first layer or component placed over the mandrel during catheter construction is the liner. The liner will become the inner wall, or lumen, of the completed catheter. Generally, the most desired features of a catheter liner are thin walls and low coefficient of friction (high lubricity). Thin walls maximize intraluminal space while keeping the overall device profile to a minimum. High lubricity reduces friction against devices and tools which pass through the catheter.
With industry-leading sizing, tight tolerances, and high-performance properties, Zeus PTFE Sub-Lite-Wall® liners are an ideal choice for a wide variety of advanced vascular catheter designs. Super-thin-walled PTFE Sub-Lite-Wall™ liners can be produced with wall thicknesses ranging from 0.001” – 0.005” (0.0254 mm – 0.127 mm), tolerances as low as ± 0.0005″ (0.0127 mm), and with uniform inside and outside diameters.
| Sub-Lite-Wall™ Liners | |
| Process | Free-Extruded |
| Material | PTFE |
| Inside Diameter (ID) | 0.002″ to 0.500″ (0.051 mm to 12.70 mm) |
| ID Tolerance +/- | 0.0005″ to 0.003″ (0.0127 mm to 0.0762 mm) |
| Nominal Wall Thickness | 0.001″ to 0.005″ (0.0254 mm to 0.127 mm) |
| Wall Tolerance +/- | 0.0005″ to 0.001″ (0.0127 mm to 0.0254 mm) |
| Cut Length | 86″ Max (2184.4 mm) |
| Surface Treatment | Etched, Tie Layer |
| Sterilization | Autoclave, EtO |
Taking our PTFE Sub-Lite-Wall™ liners a step further, Zeus StreamLiner™ Series offers the thinnest extruded catheter liners in the industry. Available in both free extruded and over-the-wire (OTW) configurations, these ultra-thin-walled liners capitalize on our proprietary extrusion technology to produce new liners with exceptional strength and functionality.
| StreamLiner™ VT | StreamLiner™ XT | StreamLiner™ OTW VT | StreamLiner™ OTW XT | StreamLiner™ OTW UT | |
| Process | Free-Extruded | Free-Extruded | Extruded Over-the-Wire (OTW) | Extruded Over-the-Wire (OTW) | Extruded Over-the-Wire (OTW) |
| Material | PTFE | PTFE | PTFE | PTFE | PTFE |
| Inside Diameter (ID) | 0.004″ to 0.120″ (0.102 mm to 3.048 mm) |
0.004″ to 0.040″ (0.102 mm to 1.016 mm) |
0.013″ to 0.0915″ (0.330 mm to 2.324 mm) |
0.013″ to 0.040″ (0.330 mm to 1.016 mm) |
0.013″ to 0.020″ (0.330 mm to 0.508 mm) |
| ID Tolerance +/- | 0.0005″ to 0.001″ (0.0127 mm to 0.0254 mm) |
0.0005″ to 0.001″ (0.0127 mm to 0.0254 mm) |
0.0005″ (0.0127 mm) |
0.0005″ (0.0127 mm) |
0.0005″ (0.0127 mm) |
| Nominal Wall Thickness | 0.00075″ (0.01905 mm) |
0.0005″ (0.0127 mm) |
0.00075″ (0.01905 mm) |
0.0005″ (0.0127 mm) |
0.0004″ (0.0102 mm) |
| Wall Tolerance +/- | 0.00025″ (0.00635 mm) |
0.00025″ (0.00635 mm) |
0.00025″ (0.00635 mm) |
0.00025″ (0.00635 mm) |
0.0002″ (0.0051 mm) |
| Cut Length | 86″ Max (2184.4 mm) |
86″ Max (2184.4 mm) |
86″ Max (2184.4 mm) |
86″ Max (2184.4 mm) |
86″ Max (2184.4 mm) |
| Surface Treatment | Etched, Tie Layer | Etched, Tie Layer | Etched | Etched | Etched |
| Sterilization | Autoclave, EtO | Autoclave, EtO | Autoclave, EtO | Autoclave, EtO | Autoclave, EtO |
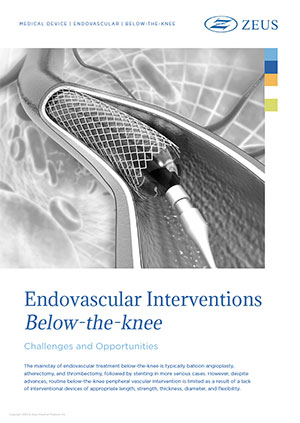
The mainstay of endovascular treatment below-the-knee is typically balloon angioplasty, atherectomy, and thrombectomy, followed by stenting in…
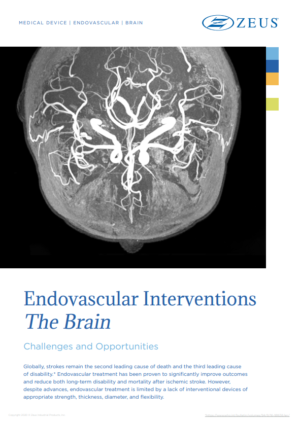
Endovascular treatment has been proven to significantly improve outcomes and reduce both long-term disability and mortality after ischemic stroke. However, despite advances, endovascular treatment is limited by a lack of interventional devices…
Polyimide (PI) tubing can be produced in a range of ID sizes with thin walls and extremely tight tolerances while still retaining its strength and pushability, enabling it to reach the smallest vasculatures. Zeus PI tubing products help you adhere to REACH and EU MDR guidelines regarding SVHC/CMR restricted substances.* When enhanced lubricity is needed, PI Glide™, a PI/PTFE composite, provides up to 25% lower coefficient of friction.
Braid and coil reinforced polyimide tubing are available for challenging applications that require enhanced torque, flexibility, kink resistance, improved pushability, or increased strength. These features help medical device engineers optimize their medical device designs and fine-tune key performance characteristics, paving the way for improved procedural outcomes and increased patient safety. Reinforced PI tubing can be produced in full-load and half-load braid patterns, as well as clockwise and counter-clockwise coiling, with various PPI (picks per inch) and WPI (wraps per inch) to meet your design requirements.
*Our thorough analytical test results indicate that no SVHC/CMR restricted substances are intentionally included in Zeus-supplied polyimide products at levels above the 0.1% threshold outlined by REACH and EU MDR.
| Polyimide Tubing | Reinforced Polyimide Tubing | |
| Process | Film-Cast | Film-Cast |
| Material | Polyimide, PI Glide™ | Polyimide, PI Glide™ |
| Inside Diameter (ID) | 0.0045″ to 0.090″ (0.1143 mm to 2.286 mm) |
0.010″ to 0.070″ (0.254 mm to 1.778 mm) |
| ID Tolerance +/- | 0.0002″ to 0.001″ (0.0051 mm to 0.025 mm) |
0.0002″ to 0.001″ (0.0051 mm to 0.025 mm) |
| Wall Thickness | 0.0004″ to 0.005″ Nom. (0.0102 mm to 0.127 mm) |
0.002″ – 0.006″ Total (0.051 mm to 0.152 mm) |
| Wall Tolerance +/- | ± 25% | ± 25% |
| Cut Length | Up to 78″ Max (Up to 1981.2 mm) |
Up to 78″ Max (Up to 1981.2 mm) |
| Surface Treatment | Tie Layer Pebax®, Vestamid® |
Tie Layer Pebax®, Vestamid® |
| Reinforcement | N/A | 304V Stainless Steel, Nitinol |
| Colors | Natural, Amber, Green, Red, Black | Natural, Amber, Green, Red, Black |
| Sterilization Methods | EtO, Gamma**, e-beam** | EtO, Gamma**, e-beam** |
** Gamma and e-beam sterilization methods are not available with PI Glide™