Medical
- Accelerate Your Prototyping
- Fast
- Easy
- Free
Medical
From material selection to custom polymer solutions, Zeus extrusion technologies get you where you need to be within the body. Our scientists and polymer engineers work with you to develop polymer solutions that are stronger, safer, and more durable to improve clinical outcomes and raise the level patient care. Continuously innovating and partnering with medical professionals and manufactures has kept us and our partners at the forefront of medical device innovation.
To meet the demands of the always evolving medical sector, Zeus has developed solutions for a broad spectrum of applications incorporating the polymer properties that matter most. Our extruded fluoropolymers, nylons, LCP, and other engineered plastics have found their way into almost every medical niche. Offering extensive levels of customization, Zeus biocompatible products can be tailored to almost any use within the body including as permanently implantable or bioabsorbable device components.
Medical Markets
Within the medical sector, Zeus serves several vascular and non-vascular sub-markets. For the human vasculature, our catheter components are used in cardio- and neurovascular applications as well as peripheral applications. In addition to these areas, Zeus products are used in cardiac rhythm management (CRM), electrophysiology (EP) and structural heart applications, as well as in renal denervation procedures. For those non-vascular associated devices, Zeus catheter componentry is found in procedures including gastrointestinal endoscopy (GI ENDO), diabetes management, oncology, brachytherapy; ear, nose, and throat (ENT); urology, neuromodulation, as well as drug delivery and cosmetic surgery.
Catheter Componentry
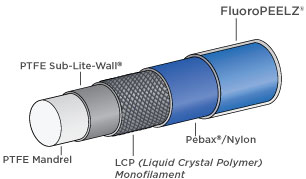
Zeus provides several key components for catheters that are crucial to their end functionality including torquability, kink resistance, and deflectability. Our PTFE extrusions are used for mandrels upon which the catheter is made, while Zeus PTFE Sub-Lite-Wall™ covers the mandrel and is etched to enable bonding to Pebax® or nylon jacketing. LCP monofilament catheter braiding provides a strength that is superior to almost all other non-metallic catheter braidings and is MRI-compatible. Zeus FluoroPEELZ™ peelable heat shrink completes the construction and provides a safe and repeatable means for removing the heat shrink after jacket reflow. Whether as fusing sleeves, liners, guide wire coverings, or introducer sheaths, Zeus catheter componentry plays an essential role in the success of these devices.
EMERGING PRODUCTS and TECHNOLOGIES
Absorv™ Bioabsorbable Extrusions
Zeus Absorv™ and our other bioabsorbable polymers can safely exist in the body for controlled lengths of time. These customized extruded device components can be made into vascular scaffolds, orthopedic screws, coatings for use in drug delivery, as well as temporary tissue scaffolds. In addition to tubing, Absorv™ can be created in biaxially and uniaxially oriented forms and in multi-layers.
Aeos™ ePTFE
Our Aeos™ ePTFE (expanded PTFE) is made using Zeus’ extrusion and expansion processing to create a product with microporous properties. The ePTFE fibrils form a matrix containing solid nodes with spaces between the nodes and fibrils that can be altered during processing. The result is a material with microporous – and breathable – properties that is highly suited to a number of medical applications. Aeos™ ePTFE can be made into membranes, tubing, monofilament (including sutures), special profiles, and laminates and can be biaxially and uniaxially oriented.
Bioweb™ Electrospun Nonwovens
Also made from PTFE, Zeus Bioweb™ nonwovens have microporous properties similar to Aeos™ ePTFE. These custom composites can be used to create implantable structures such as stent coverings including balloon-expandable and self-expanding stents and mimic the extracellular matrix to speed healing. Bioweb™ novel encapsulations prevent stent struts from contacting the luminal wall and thus reduce their influence on the healing process.
Custom Polymer Solutions for Medical Markets
At Zeus, our leading polymer scientists, engineers, and technical account managers are ready to assist with all your project needs. Customized solutions are available via phone, e-mail, or video conferencing. In-person consultations are also available on the Zeus campus or we will come to you. Our Z-Team is also available for a more thorough start-to-finish engagement.
Contact us today or call toll-free in the US 1-800-526-3842 to learn more about these polymer solutions or any of our other medical device products. Internationally, you can reach us at +1-803-268-9500.