Projects
Filter by:
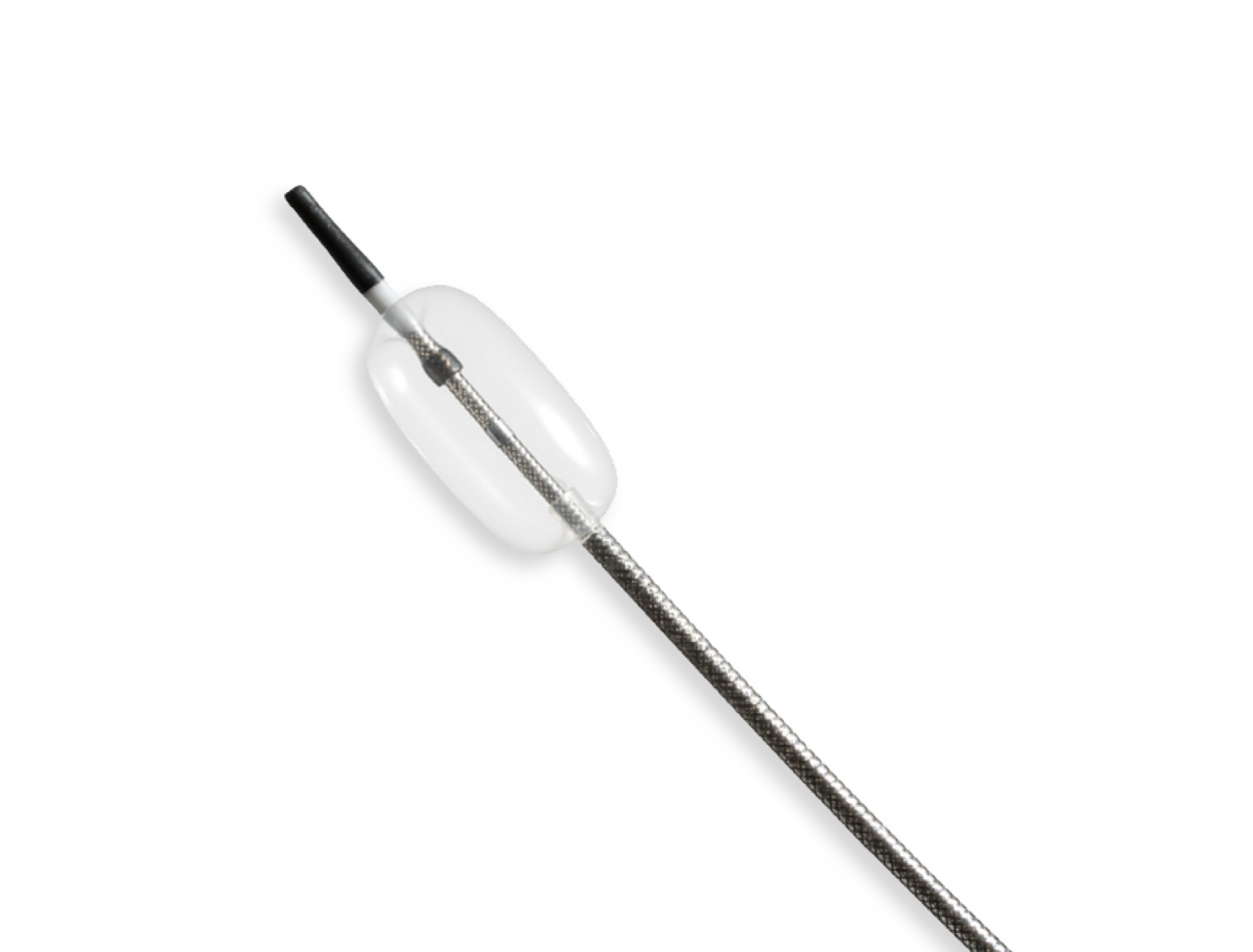
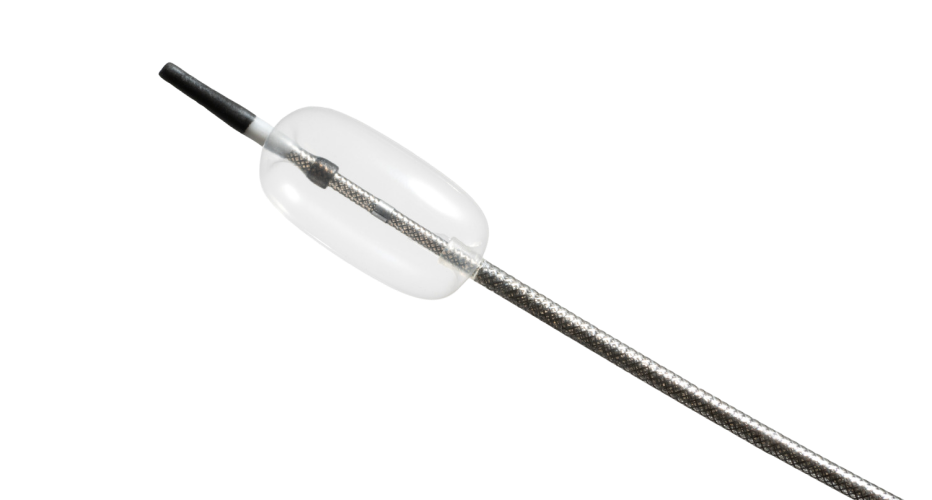
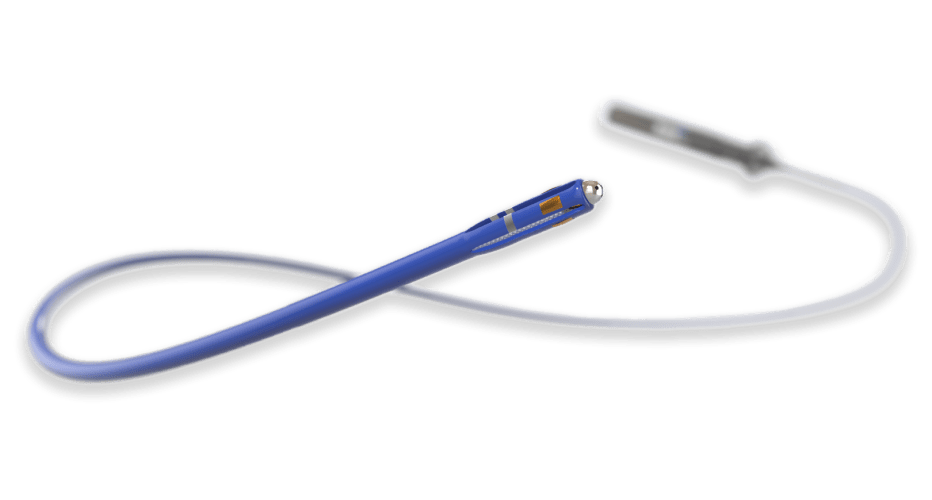
Compliant Balloon Microcatheter
In collaboration with the customer’s R&D engineers, we developed a highly specialized microcatheter using a compliant occlusion balloon.
To meet exacting requirements, a custom co-axial braided catheter shaft was designed with exceptional pushability, lubricity, and kink resistance.
The unique design featuring a sub-3Fr catheter profile, flexible body, atraumatic tip, and compliant balloon allows for maximum patient compatibility.
Compliant balloon materials often require advanced bonding techniques to create a robust, leak-proof bond to the catheter body. Typically, this bonding creates an increased profile across the bond area. However, for this project to meet clinical requirements, the device needed a smooth, homogenous profile.
In close collaboration with the customer’s R&D engineers, we developed a highly customized balloon bonding process. A specialized reinforced braided catheter shaft was engineered enabling a device design with exceptional pushability, lubriciousness, and kink resistance.
We improved the customer’s development schedule while maintaining all design requirements. Yields of 98%+ were achieved through rigorous leak testing.
“A genuine and highly collaborative partner, and a natural extension of our R&D and production team.”
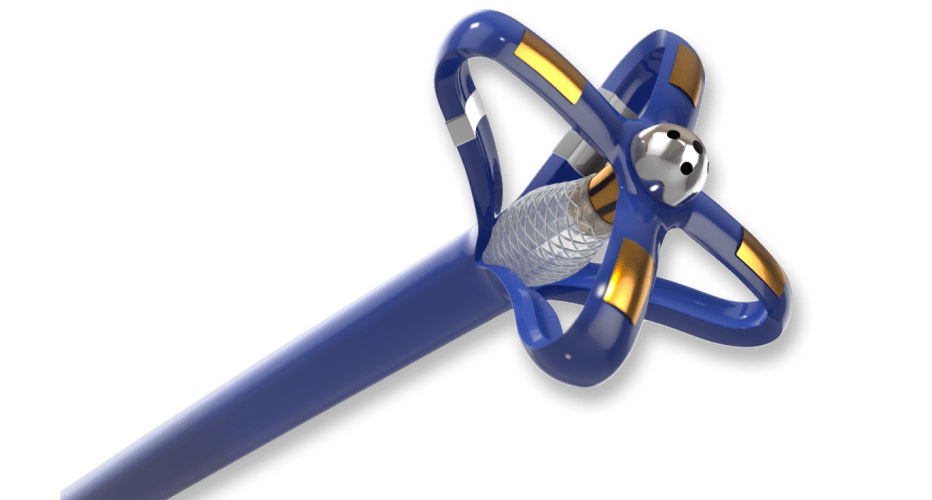
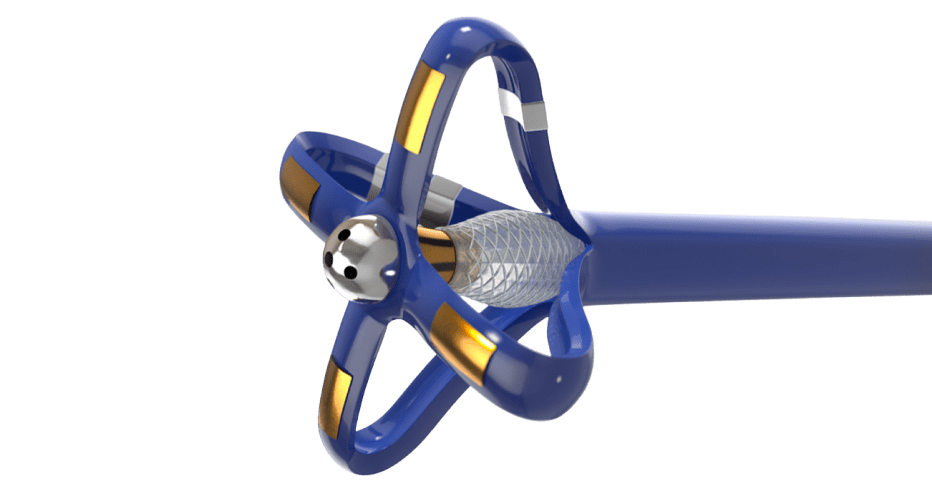
Steerable Delivery Catheter
Due to strict planarity for precise placement of an implant, the customer required an innovative catheter design solution.
We developed a unique and highly specialized PTFE liner solution to aid the manufacture of the steerable catheter.
A custom braided catheter shaft integrates coils and laser-cut hypotubes to provide excellent steerability, flexibility, and torque response.
Every catheter engineer faces challenges with planarity of the final catheter. Keeping the articulated section of the catheter in-plane is critical for the physician to accurately and consistently use the device. Many variables during the manufacturing process can negatively affect the planarity.
We collaborated with our partners at Zeus to develop a unique and highly customized PTFE liner solution to aid the manufacture of the steerable catheter. This highly customized liner reduced manufacturing variation and delivered more consistent finished devices.
We supported the customer’s commercial product launch while minimizing variation in a robust manufacturing process. The capability to design and develop a custom liner for large-scale manufacturing was key to the program’s success.
“Their drive to understand our design needs and develop flexible manufacturing solutions is key to our NPI success.”
Non-Compliant Balloon Catheter
We designed, developed, and manufactured a non-compliant balloon catheter with specially placed markers to allow the physician unparalleled visualization before deploying the stent.
Balloon catheters typically incorporate inflation and deflation lumens to operate. These additional lumens can negatively impact catheter device flexibility because of the complex internal geometry. To precisely place the stent, the cardiologist requires flexible balloon catheters along with clear visualization under fluoroscopy.
We partnered with the customer to develop a novel and “industry-first” catheter reinforcement method. The resulting solution allowed the inflation and deflation lumens to function without impacting the catheter’s flexibility.
Our close collaboration with the customer across all stages of the program development led to the device completing pre-clinical trials in record time. It is now in validation before entering clinical trials for human use.
“Outstanding in their ability to help us develop an optimal manufacturing process. Always listening to our needs and responding to meet milestones, they are a genuine partner.”
Mapping and Ablation Catheter
Electrophysiology catheters are among the most complex devices in interventional medicine. Precise electrical components are packaged into state-of-the-art catheters to allow physicians exceptional control during the procedure.
Contracted by a highly ambitious medical device company, we developed a novel, irrigated electrophysiology catheter for diagnostic mapping and ablation to aid heart function.
A complex braid reinforced catheter shaft was designed to provide precise location control at the distal tip.
Electrophysiology catheters are a balance between sensitive electronics and high-tech catheters. Striking the right balance presents a key design challenge when packaging these components into a functional device.
In collaboration with the customer, our senior engineering team applied their technical expertise to provide critical “design for manufacturability” input. Design features incorporate a flexible construction to improve contact stability and focal mapping and ablation in a single catheter.
A novel but cost-effective design for this low-power, temperature-controlled ablation catheter was developed ahead of schedule, allowing the customer to prepare for regulatory approval in record time.
“Their talented engineering team quickly understood our design requirements and implemented a robust product development process. They are a genuinely collaborative development partner.”
Below-the-knee Stent Delivery Catheter
Good push-ability is often traded for a thinner profile in catheter design, particularly for below-the-knee procedures.
For this project, our customer required a stent delivery catheter with both performance characteristics to enable access deep in the popliteal artery.
The requirement for this device was to have an ultra-low catheter profile (sub 0.004″/0.10 mm) combined with enhanced push-ability to deliver treatments to areas of the body, which are typically difficult to reach.
In collaboration with our wider engineering team at Zeus, we specified the optimum polymer profile and designed a custom reinforcement method to strengthen the catheter without sacrificing wall thickness. Functional prototypes were quickly developed for pre-clinical trial testing using our rapid prototyping capability.
Pre-clinical test results indicate the design performs with superior efficacy to precisely navigate below-the-knee and accurately place stents in previously thought impossible locations. Based on these excellent results, validations are underway.
“Their expertise in catheter design and their prototyping capability was crucial. Creating a holistic solution allowed us to optimize our device to meet clinical demands.”