Aeos™ ePTFE Suture Monofilament (ASM) is Class VI approved, non-absorbable, and can be used as a permanent implant. Needles can be swaged onto ASM down to a 1:1 needle-to-suture ratio. Available license-free and in bulk quantities.
The operating table, that is. Zeus Aeos™ ePTFE extrusions are made by expanding PTFE under controlled conditions during manufacturing. The result is a microporous product that is flexible and strong. The structure of ePTFE supports new tissue ingrowth. Chemically inert, this material provokes little to no immune response and results in less scarring. Aeos™ sutures maintain a high tensile strength and perform well in stressful anatomical environments. ASM is USP Class VI approved and non-toxic.
To learn more about our full line of medical device components, visit us at MD&M Minneapolis 2018 at the Minneapolis Convention Center, October 31 – November 1. We look forward to meeting you at Booth 1805.
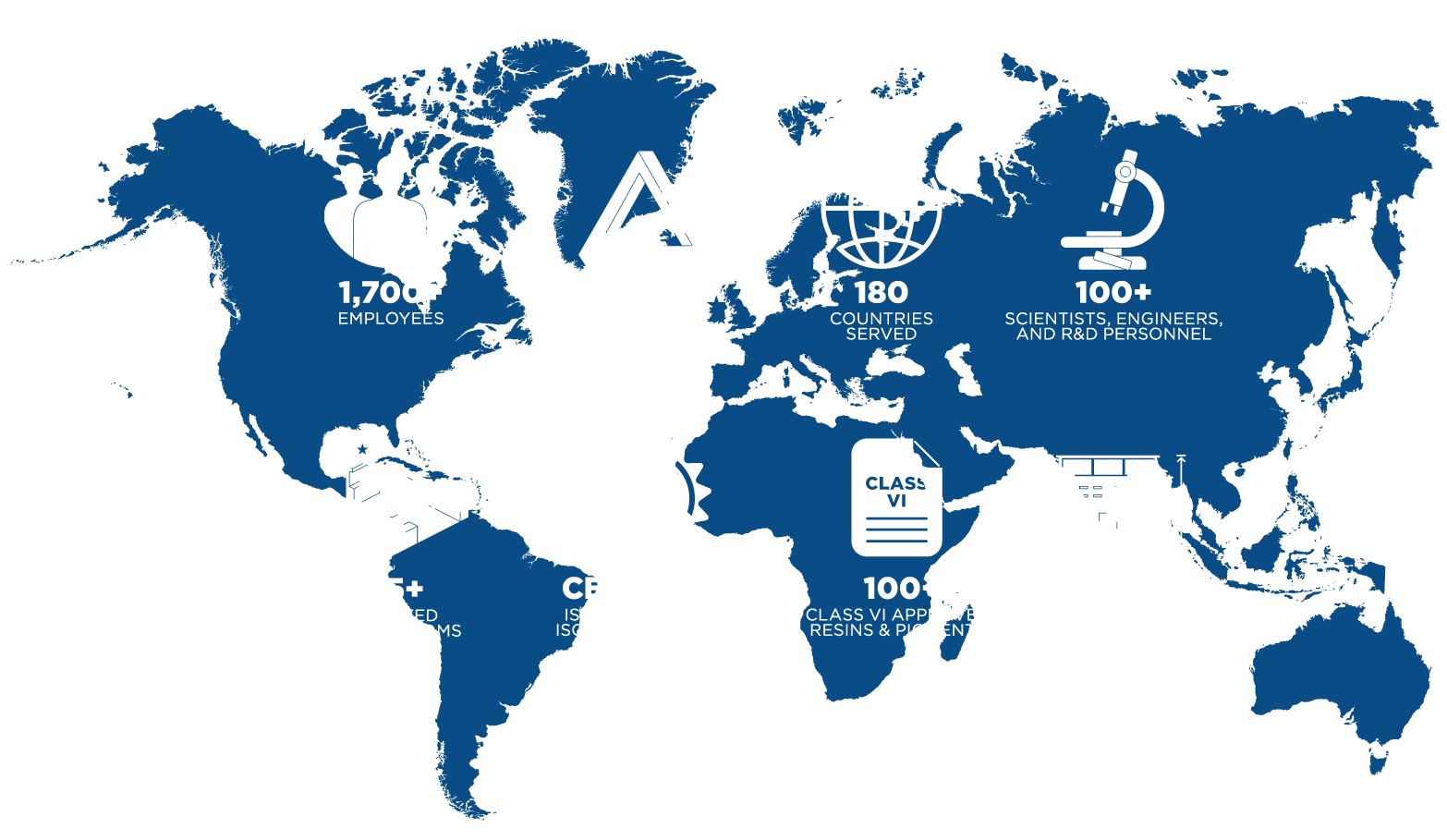
Zeus’ history and reputation of partnering with manufacturers and research and development groups is long well-recognized. Today, companies – whether well-established or start-up – know that when they come to us, we deliver!